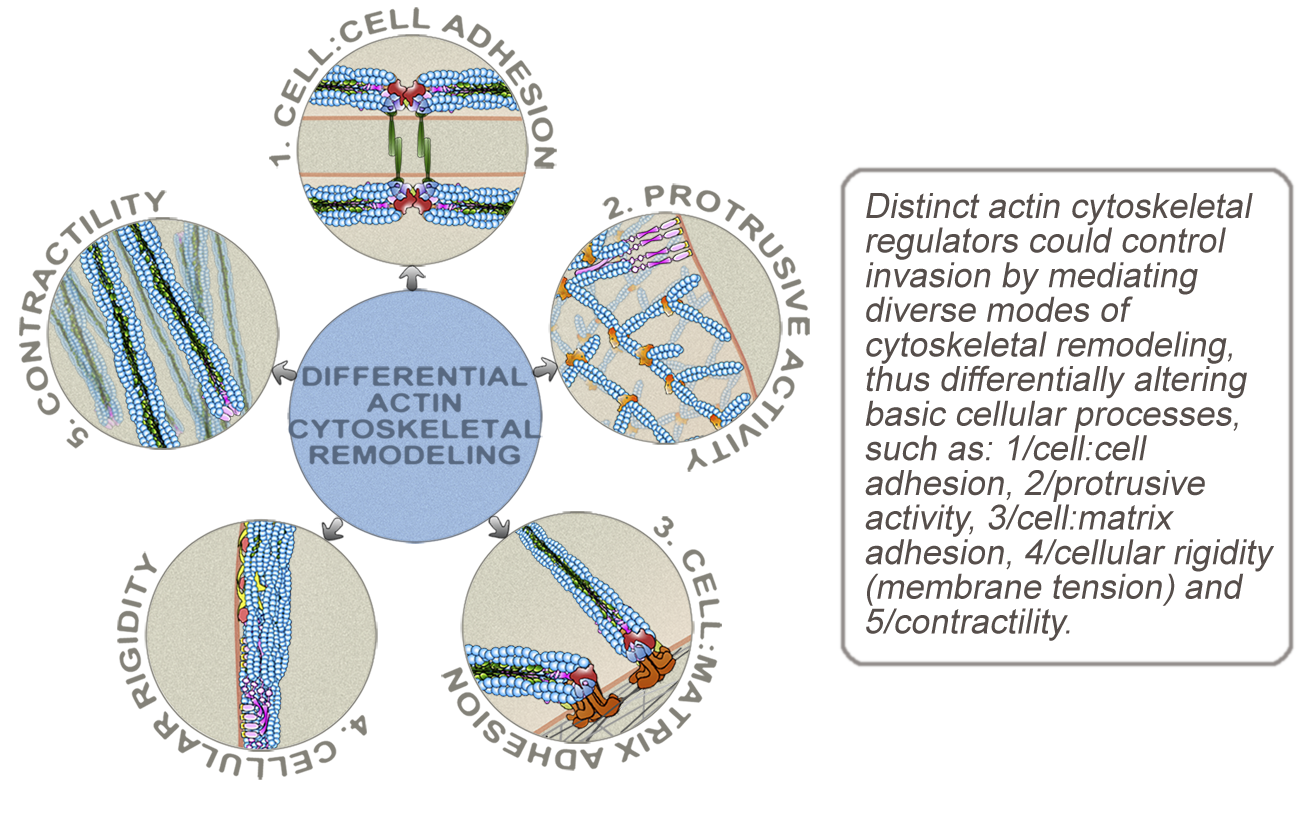
 Invasion is an integral process to tumor metastasis in different types of cancers, particularly in carcinomas – cancers from epithelial origin. In most carcinomas, invasive behavior is dependent on cellular activities that enable cancer cells to overcome the structural restraints of tissue organization, migrate as single cells or as large or small cohorts of cells through stroma, and finally intravasate into the vasculature. Considering that the spreading of tumors to secondary organs is one of the primary causes of death in cancer patients, therapeutic regimens targeting cancer cell invasion would be extremely valuable for cancer management and treatment. However, designing such therapies is very challenging due to the morphological and behavioral plasticity of cancer cells, which allows their adaptation to various cellular and microenvironmental changes. Therefore, understanding cancer cell plasticity is a significant step toward developing effective therapeutic strategies. An important aspect of cancer cell plasticity is the continuous integration of changes in extracellular cues with changes in cellular processes. In the lab, we take a comprehensive approach to study the role of the actin cytoskeleton in the integration of extracellular cues and in altering aspects of cell shape and behavior that are critical for invasion.
Invasion is an integral process to tumor metastasis in different types of cancers, particularly in carcinomas – cancers from epithelial origin. In most carcinomas, invasive behavior is dependent on cellular activities that enable cancer cells to overcome the structural restraints of tissue organization, migrate as single cells or as large or small cohorts of cells through stroma, and finally intravasate into the vasculature. Considering that the spreading of tumors to secondary organs is one of the primary causes of death in cancer patients, therapeutic regimens targeting cancer cell invasion would be extremely valuable for cancer management and treatment. However, designing such therapies is very challenging due to the morphological and behavioral plasticity of cancer cells, which allows their adaptation to various cellular and microenvironmental changes. Therefore, understanding cancer cell plasticity is a significant step toward developing effective therapeutic strategies. An important aspect of cancer cell plasticity is the continuous integration of changes in extracellular cues with changes in cellular processes. In the lab, we take a comprehensive approach to study the role of the actin cytoskeleton in the integration of extracellular cues and in altering aspects of cell shape and behavior that are critical for invasion.
In addition to genetic and epigenetic changes that alter the expression of actin remodeling regulators, the architecture of the actin cytoskeleton is responsive to changes in extracellular cues, such as extracellular matrix, growth factors, and inflammatory cytokines. Moreover, remodeling of the actin cytoskeleton in response to changes in extracellular cues could alter basic cellular processes, such as cell:matrix and cell:cell adhesion, contractility, protrusive activity, and cellular rigidity. Invasive behavior can be influenced by the amplitude and direction of change in a variety of cellular processes. In the lab, we are focused on understanding how distinct modes of actin cytoskeletal reorganization, induced by intracellular and microenvironmental signals, can cause simultaneous changes in multiple basic cellular processes and how several different combinations of these changes could promote invasion. The overall objective is to identify distinct minimal sets of changes in basic cellular processes sufficient to promote invasion and to identify how these changes are induced by remodeling of the actin cytoskeleton.
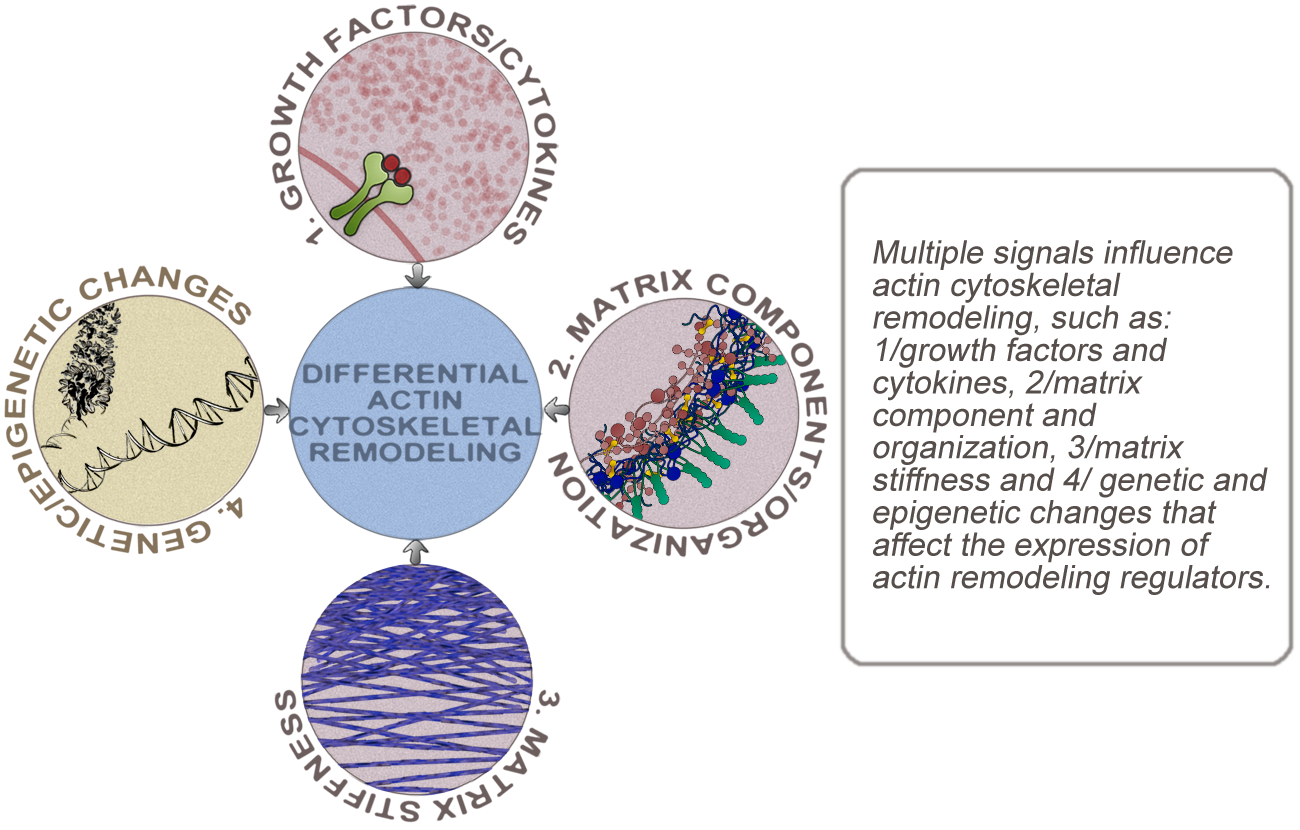 Ultimately, the goal is to characterize the clinical significance of actin cytoskeletal remodeling in cancer. Given the collaborative, antagonistic and sometimes redundant roles of actin regulators in shaping the cytoskeletal architecture, analysis of the expression of any single regulator as a means to identify an invasion 'biomarker', is unlikely to be meaningful. However, multivariate analysis of the expression of a group of functionally coupled actin regulators could identify expression patterns that correlate with invasiveness. Using a bioinformatic approach to characterize these expression patterns, together with analyses of clinical samples will allow identification of actin regulators that are associated with tumor progression and will give insight into how aberrations in cellular changes that are mediated by these regulators contribute to invasion.
Ultimately, the goal is to characterize the clinical significance of actin cytoskeletal remodeling in cancer. Given the collaborative, antagonistic and sometimes redundant roles of actin regulators in shaping the cytoskeletal architecture, analysis of the expression of any single regulator as a means to identify an invasion 'biomarker', is unlikely to be meaningful. However, multivariate analysis of the expression of a group of functionally coupled actin regulators could identify expression patterns that correlate with invasiveness. Using a bioinformatic approach to characterize these expression patterns, together with analyses of clinical samples will allow identification of actin regulators that are associated with tumor progression and will give insight into how aberrations in cellular changes that are mediated by these regulators contribute to invasion.
